Maxime carried out his research in the IDeAS team at the Softmat laboratory in collaboration with TBI.
On 26th October, he defended his thesis entitled: “Polymer surfaces with tunable enzymatic activity”
While the exploitation of plant biomass represents a great opportunity for the production of high value-added molecules or biofuels, it is very energy intensive and remains very limited in terms of yield and diversity of products extracted. One way to overcome these limitations is to look to nature for inspiration. Indeed, some microorganisms express complex cocktails within protein structures immobilized on the outer surface of their membranes in order to deconstruct and metabolize this biomass.
The aim of his work is to provide an original approach to one of the questions raised by these processes of enzymatic deconstruction of a complex, insoluble substrate: how do these enzymes behave at the solid/liquid interface and how does their spatial proximity affect their action?
To answer these questions, he has developed a unique approach to immobilize enzymes on an elastomer surface. By mechanically stretching the surface, we can vary the surface density of the enzymes and thus the distance between them.
In the first part, he characterizes all the elements used in this project. First, the four synthetic or commercial elastomer surfaces used for enzyme immobilisation are characterized both at the molecular level and in terms of their surface state. Then, the enzyme in question, a xylanase, is immobilized on the elastomers via a pair of Jo and In proteins, allowing oriented and covalent immobilisation. The aim is to ensure the activity of our immobilized enzymes.
The second part of his thesis focuses on the functionalisation of the surfaces studied. First, the necessity of this functionalisation is highlighted, then the method used is described in detail. Finally, we describe the characterisation method developed to highlight the functionalisation of the different surfaces.
The third part of his thesis characterises the grafting of proteins onto our elastomer surfaces. First, the protein Jo. Its grafting is achieved by a Michael thiol-maleimide reaction. This step required the implementation of a wide range of complementary approaches to finely characterize a complex process involving the immobilisation of a small amount of protein in a monolayer on a smooth surface. The xylanase, expressed in fusion with In, is then grafted onto the surface by covalent association of the Jo/In pair. This immobilisation step will again be characterized and the enzymes immobilized on the surface will be quantified using a method developed specifically for this project.
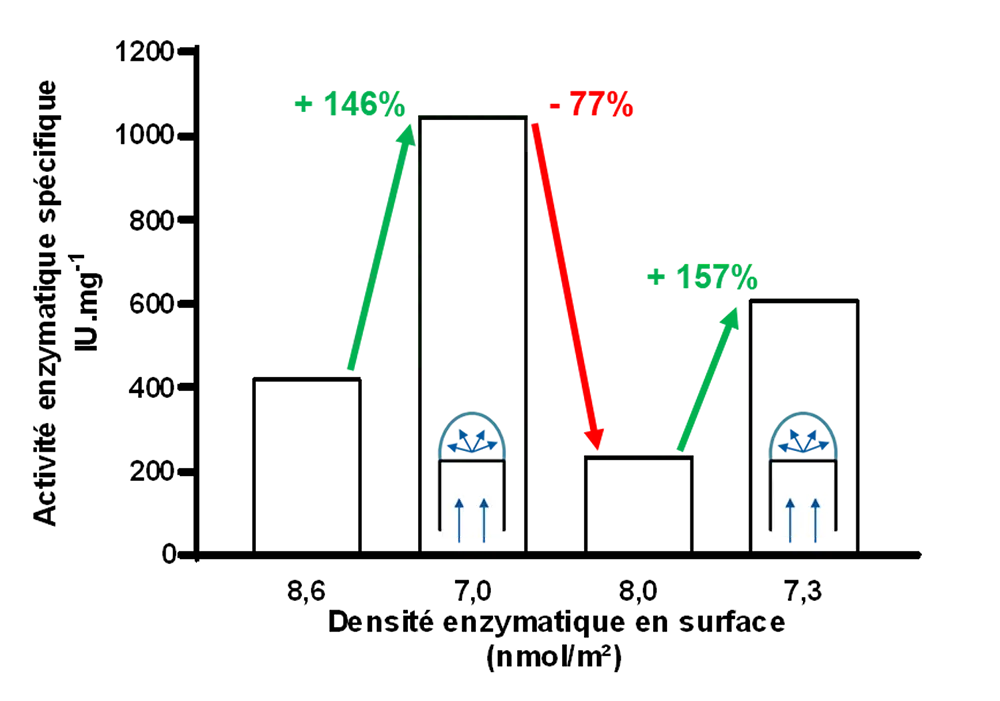
The final part of his thesis investigates the effect of the distance between immobilized enzymes on their specific activity. The grafted surfaces, arranged on a stretching tool specifically developed during the course of this work, were stretched in increasing proportions, thereby increasing the distance between the enzymes. He shows that a decrease of enzyme density on the surface, and therefore an increase in the distance between enzymes, leads to a proportional increase in their specific activity, approaching that of free enzymes in solution.
He also highlights the technical limitations of this approach and proposes some development solutions to exploit the full potential of this strategy.
Highlights of the thesis:
- Maxime is co-author of an article published in the journal Biotechnology Advance (Impact Factor=16).
Congratulations to Maxime for the quality of his work!
